Post Electrophoretic Analysis
An Overview of Northern and Southern Blotting
A DNA or RNA probe will selectively hybridize with nucleic acid molecules of complementary sequence in a sample. A labeled nucleic acid molecule of known sequence can facilitate detection of any complementary molecules in an unknown sample. This is the basis of the RNase protection assay, and PCR amplification, among other techniques. In theory, labeled nucleic acid molecules could act as specific "stains" for DNA or RNA species in gels. Only complementary fragments would be "stained." However, specific hybridization requires nucleic acid polymers of twenty-five or more bases, which are too large to diffuse rapidly into a gel.
In order to circumvent this problem, a method was devised to "print" an electrophoretic pattern onto a solid support, preserving the positional information from a gel, but removing the matrix. This process, blotting, was first publicized by Southern (1975). Southern demonstrated that DNA could be electrophoretically fractionated, transferred to nitrocellulose, and then probed with radioactively labeled DNA sequences, which would hybridize to their cognates bound to the membrane.
The result dubbed a "Southern Blot", reveals a pattern of bands showing the size and relative amount of DNA molecules containing the probe sequence. Soon thereafter RNA was blotted successfully (Northern Blotting) and protein (Western Blotting). In all cases the advantage gained by blotting onto a membrane was the immobilization of the electrophoretic pattern, rendering the molecules in that pattern accessible to macromolecular probes.
Transfer Techniques
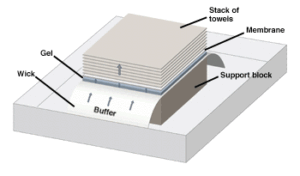
Capillary blotting. Transfer buffer is drawn up the wick, through the gel and membrane, and into the dry stack of towels. The flow of buffer elutes the nucleic acid molecules from the gel onto the membrane, preserving the band pattern.
Blots are created by laying a membrane over one face of the gel and then creating a flow that carries the molecules in the gel onto the membrane. The two most common methods used for Northern and Southern blotting are capillary flow and vacuum transfer. Electroblotting is also used occasionally, although it requires special care to prevent the crushing or melting of the agarose gel.
Capillary flow transfers are carried out in a dish of buffer (see figure below). The gel is placed on porous support (generally filter paper or a sponge) which holds the gel above the buffer while allowing the buffer to flow up to it. The membrane is placed on top of the gel, and a stack of absorbent paper is placed over it. A weight is placed on top of the stack to ensure continued close contact of all components, and the entire assembly is left to stand for 12-16 hours (i.e. overnight). During this period, the buffer is wicked out of the gel through the membrane and onto the dry filters above. As buffer flows into the gel from the tank below, the nucleic acids in the gel are carried upward, until they meet the membrane. Conditions are chosen such that binding to the membrane is favored, so molecules of nucleic acid are trapped there. Generally, 80-90% of the molecules in the gel can be recovered on the membrane by this procedure.
Vacuum blotting also uses a flow of buffer to elute bands onto the membrane, but it uses a vacuum instead of capillary action to create the flow. The membrane is placed on a porous support, the gel is placed over it, a vacuum is applied and the buffer is added to submerge the gel. This type of blotting is quite fast (1-3 hrs.), and gives excellent recoveries. Its only drawback is the need for specialized apparatus.
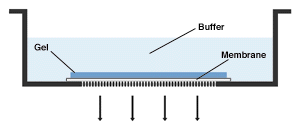
Vacuum blotting. Buffer is drawn down through the gel and membrane by a vacuum in the lower chamber, eluting bands from the gel onto the membrane.
Electroblotting of DNA or RNA from agarose gels is most often carried out in a "semi-dry" apparatus, between solid flat electrodes of metal or graphite. In this technique, the potential is applied perpendicular to the gel, causing the nucleic acid fragments to eletroelute onto the membrane. With agarose, special care must be taken not to crush, distort or melt the gel during transfer. Electroblotting is far more popular for polyacrylamide gels in which capillary or vacuum techniques fail due to the smaller pore size.
In the methods given below for Northern and Southern blotting, directions are given for setting up capillary blots. For instructions on vacuum or electro-blotting, see the protocols supplied by manufacturer's of these devices.
Membranes
There are two popular membrane materials used in nucleic acid blotting. These materials are nitrocellulose (including supported nitrocellulose), and nylon (charged and uncharged). Nitrocellulose is the traditional support medium, and it is still employed by many researchers. In a high salt aqueous environment, nitrocellulose will bind to nucleic acids tightly but non-covalently. After transfer, nucleic acid binding is stabilized by baking the filter for 1 hour. The baked filter can be stripped and reprobed, but because the bound nucleic acid is not covalently linked to the membrane, significant signal loss can occur. A major drawback to nitrocellulose membranes is that they are quite fragile, requiring very careful handling to preserve the blot intact.
Nylon filters have gained great popularity since their introduction, their most obvious advantage over nitrocellulose being their durability. Nylon filters can be handled roughly with no physical damage. The material withstands prolonged exposure to alkali, which allows use of the convenient alkaline transfer technique. Nylon is blocked by SDS, which simplifies the pre-hybridization steps.
Nucleic acids can be covalently linked to nylon membranes through controlled exposure to UV light. The resulting blot may be stripped and reprobed many times with minimal loss of signal. Nylon membranes are available in uncharged form, or with immobilized positive charges on their surfaces. Positively charged nylon has a higher affinity for nucleic acids, which are anions, than does neutral nylon. Positively charged nylon will bind nucleic acid semi-permanently after alkaline blotting without UV cross-linking. However, in some cases, charged nylon may give higher backgrounds than uncharged membranes.
NEXT TOPIC: Northern Blotting
- UV Shadowing
- Uneven Staining
- Staining Proteins Immobilized on Membranes
- Staining Protein Gels with Coomassie Blue
- Southern Blotting
- Smeared Bands
- Silver Staining Protein Gels
- Silver Staining DNA Gels
- Protein Fixation on Gels
- Post-Electrophoretic Visualization with Nuclistain
- Overview of Western Blotting
- Northern Blotting
- Method for Western Blotting
- Mechanism of Immunostaining
- Mechanism of Immunostaining
- Immunostaining with Alkaline Phosphatase
- Guide Strip Technique
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Enzyme Linked Immunosorbent Assay (ELISA)
- Coomassie Blue Stain- Troubleshooting
- Blotches on Gel
- Autoradiography
- Autoradiographic Enhancement with Autofluor
- An Overview of Northern and Southern Blotting
- Alkaline Blotting
