Gel Electrophoresis of Proteins
Immuno-Electrophoresis / Immuno-Diffusion
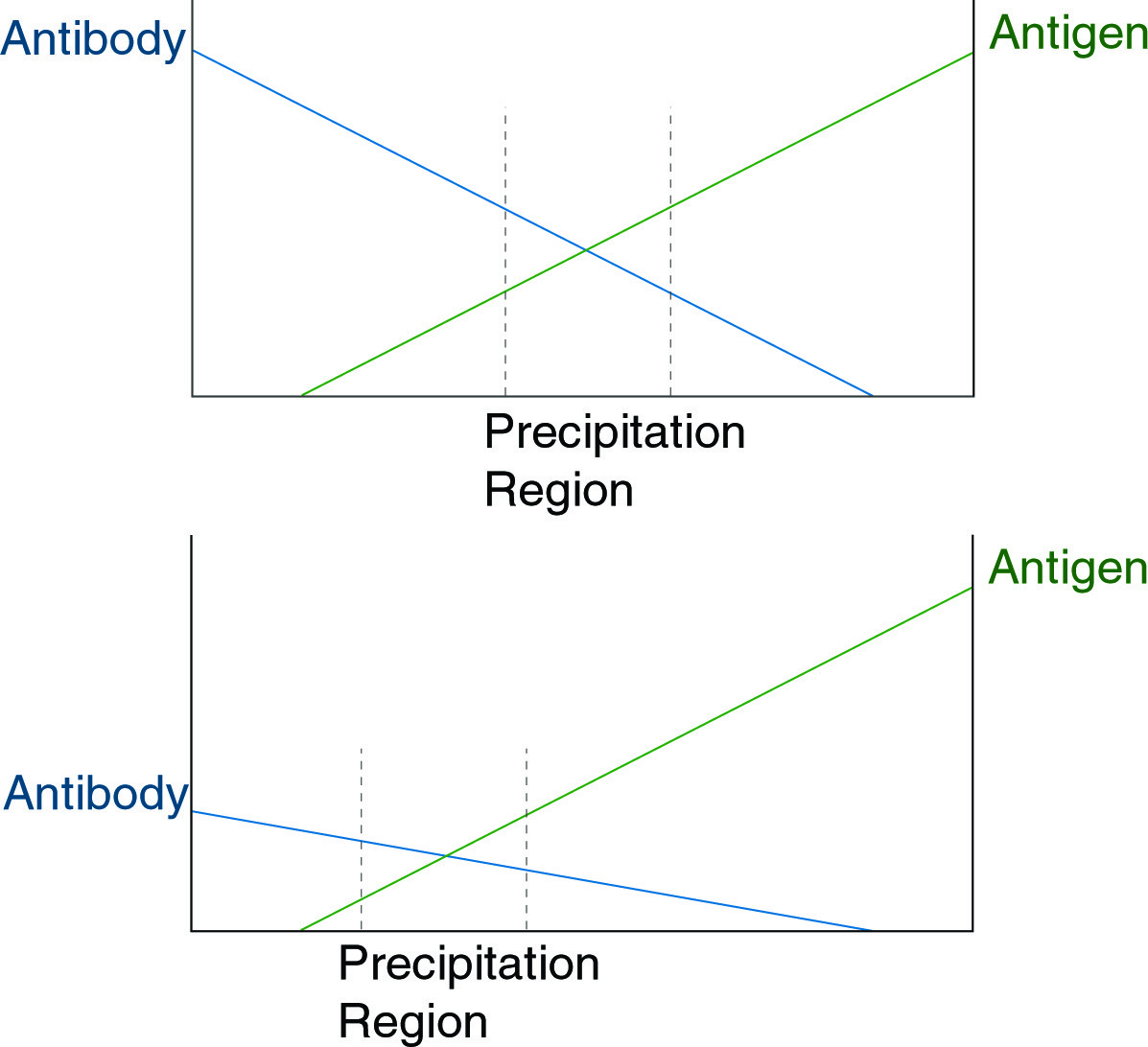
When present at approximately equal concentration, antigen and antibody will precipitate as an aggregate. The figure above illustrates a principle underlying the usefulness of immunodiffusion, that with shifts in antibody concentration, a corresponding shift in the region of precipitation will occur.
Antibodies are produced by the immune system in response to foreign macromolecules. Each antibody binds specifically to one feature (epitope) on one macromolecule (antigen). This allows the use of antibodies for the detection and quantitation of specific proteins in complex mixtures. Antibodies are generally isolated from animal serum, unless they are produced from tissue culture as monoclonals. The serum must be titrated to determine antibody concentration and specificity. Concentration determinations are often needed for antigen mixtures as well. Immunodiffusion and immunoelectrophoresis are useful techniques for these purposes.
If antibody and antigen are present in solution at approximately equal concentrations, they form an aggregate, which precipitates. This precipitate can be dissolved by the addition of an excess of antibody or of antigen (see figure below).
When present at approximately equal concentration, antigen and antibody will precipitate as an aggregate. The figure above illustrates a principle underlying the usefulness of immunodiffusion, that with shifts in antibody concentration, a corresponding shift in the region of precipitation will occur.
In a gel matrix, if a gradient is established in which antibody concentration decreases linearly in a given direction while antigen concentration increases in the same direction, a precipitate will form a band perpendicular to this direction at the point of approximately equal concentration. This is the basis of both immunodiffusion and immunoelectrophoresis. In both techniques, a gradient of antigen, or of antibody and antigen, establishes a line of precipitate in the gel. The position of the line is indicative of the concentration of antigen or antibody in the sample and may be calibrated against standards. The figures below show different variations of the immunodiffusion assay.
Radial Immuno Diffusion
- Melt 1g agarose in 100ml PBS.
- Spread 1ml of this solution on a glass plate or microscope slide. Allow to dry. This pre coats the slide and allows the gel poured in the next step to adhere to the slide.
- Pour enough of the agarose solution onto the pre-coated slide to cover it to a depth of 1 - 2 mm. Allow this to cool until gelled.
- Punch wells in the gel with a 2 - 3 mm punch. The end of a 1ml glass pipette works well.
- Place antigen and antibody solutions in adjacent wells and allow plate to diffuse 24 hours. It will be necessary to run multiple dilutions of sample and antibody to determine optimum concentrations of each.
Immuno-Electrophoresis
Agarose gels have a small number of fixed charges, which cause a phenomenon known as electroendosmosis. EEO causes a slow net flow of water through the gel away from the positive electrode. At a pH of 8.5, antibodies are nearly uncharged, and their slow electrophoretic migration is nullified by the EEO flow through the agarose. Most other proteins are highly charged at pH 8.5 and will migrate through an agarose gel. If antiserum is included in the gel matrix, immunoprecipitates will form, in a pattern dependent upon the antigen concentration. This technique, immuno-electrophoresis, is much faster than immuno-diffusion and has been applied in a variety of geometries, to analyze simple or complex samples.
"Rocket" Immuno-Electrophoresis
- Cast a 1% agarose gel, in buffer at pH 8.6 containing typically 1% of the desired antiserum.
- Load antigen solutions into wells punched at one end. Run gel with wells closest to the negative electrode.As the antigen proteins enter the gel, they form a concentration gradient, which at some point gives the proper concentration for precipitation with the antibody in the gel. The more concentrated the antigen, the further it must run to be diluted to precipitable levels. The result is that each sample gives a “Rocket”, the length of which is proportional to the concentration of antigen in the sample.
- Precipitates can be stained with Coomassie Blue R-250 and detection limits are generally sufficient to quantitate 0.1 - 0.2 g/ml of antigen.
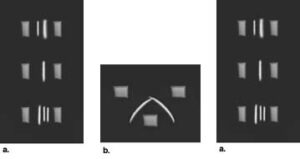
Crossed immunoelectrophoresis of antigens and antiserum. In the first dimension, proteins are separated by standard electrophoresis. The separated proteins are then run into the second dimension gel at an angle of 90° from the first dimension. The second dimension gel contains antiserum, generating an immunoprecipitate pattern like the one shown.
Mixtures containing multiple antigen species which cross-react with the same antiserum may be analyzed by running them first on an analytical gel, then cutting a strip from that gel and laying it in a slit cut into the immuno-electrophoresis gel to form a large well. The result is a pattern that shows the positions of strongly reacting antigen species.
NEXT TOPIC: Isoelectric Focusing
- Sample Preparation for SDS-PAGE
- Sample Preparation for Native Protein Electrophoresis
- Peptide Mapping
- Native Protein Electrophoresis
- Measuring Molecular Weight with SDS-PAGE
- Isotachophoresis
- Isoelectric Focusing
- Immuno-Electrophoresis / Immuno-Diffusion
- Gel Preparation for Native Protein Electrophoresis
- Gel Electrophoresis of RNA & Post Electrophoretic Analysis
- Denaturing Protein Electrophoresis: SDS-PAGE
- Casting Gradient Gels
- Activity Stains
