Gel Electrophoresis of Proteins
Denaturing Protein Electrophoresis: SDS-PAGE
In their native form, proteins fold into a variety of shapes, some compact, some elongated. The rate of migration of native proteins through a sieving medium is therefore more a reflection of their relative compactness, and less an accurate measure of molecular weight. Denaturing the proteins nullifies structural effects on mobility, allowing separation on a true charge/mass ratio basis. It also separates subunits in multimeric proteins, allowing analysis of large, complex aggregates.
The most commonly used denaturant is sodium dodecyl sulfate (SDS). SDS is an amphipathic surfactant. It denatures proteins by binding to the protein chain with its hydrocarbon tail, exposing normally buried regions and coating the protein chain with surfactant molecules. The polar head group of SDS adds an additional benefit to the use of this denaturant. Proteins solubilized in SDS bind the detergent uniformly along their length to a level of 1.4g SDS/g protein. This creates a charge/mass ratio which is consistent between proteins. For this reason, separation on a polyacrylamide gel in the presence of SDS occurs by mass alone.
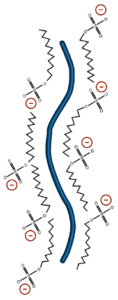
SDS is the most commonly used detergent in protein electrophoresis.
Treatment with SDS creates a uniform charge-to-mass ratio between different proteins. SDS PAGE offers a rapid and relatively accurate way to determine protein molecular weights. Masses determined by SDS-PAGE are usually accurate within 5-10%, although occasionally proteins may retain enough secondary structure or contain sufficient charged groups to migrate anomalously. The migration of histones, which carry a strong intrinsic charge, is an example of this phenomenon.
NEXT TOPIC: Sample Preparation for SDS-PAGE
- Sample Preparation for SDS-PAGE
- Sample Preparation for Native Protein Electrophoresis
- Peptide Mapping
- Native Protein Electrophoresis
- Measuring Molecular Weight with SDS-PAGE
- Isotachophoresis
- Isoelectric Focusing
- Immuno-Electrophoresis / Immuno-Diffusion
- Gel Preparation for Native Protein Electrophoresis
- Gel Electrophoresis of RNA & Post Electrophoretic Analysis
- Denaturing Protein Electrophoresis: SDS-PAGE
- Casting Gradient Gels
- Activity Stains
