Post Electrophoretic Analysis Articles
Denaturing Polyacrylamide Gel Electrophoresis of DNA & RNA
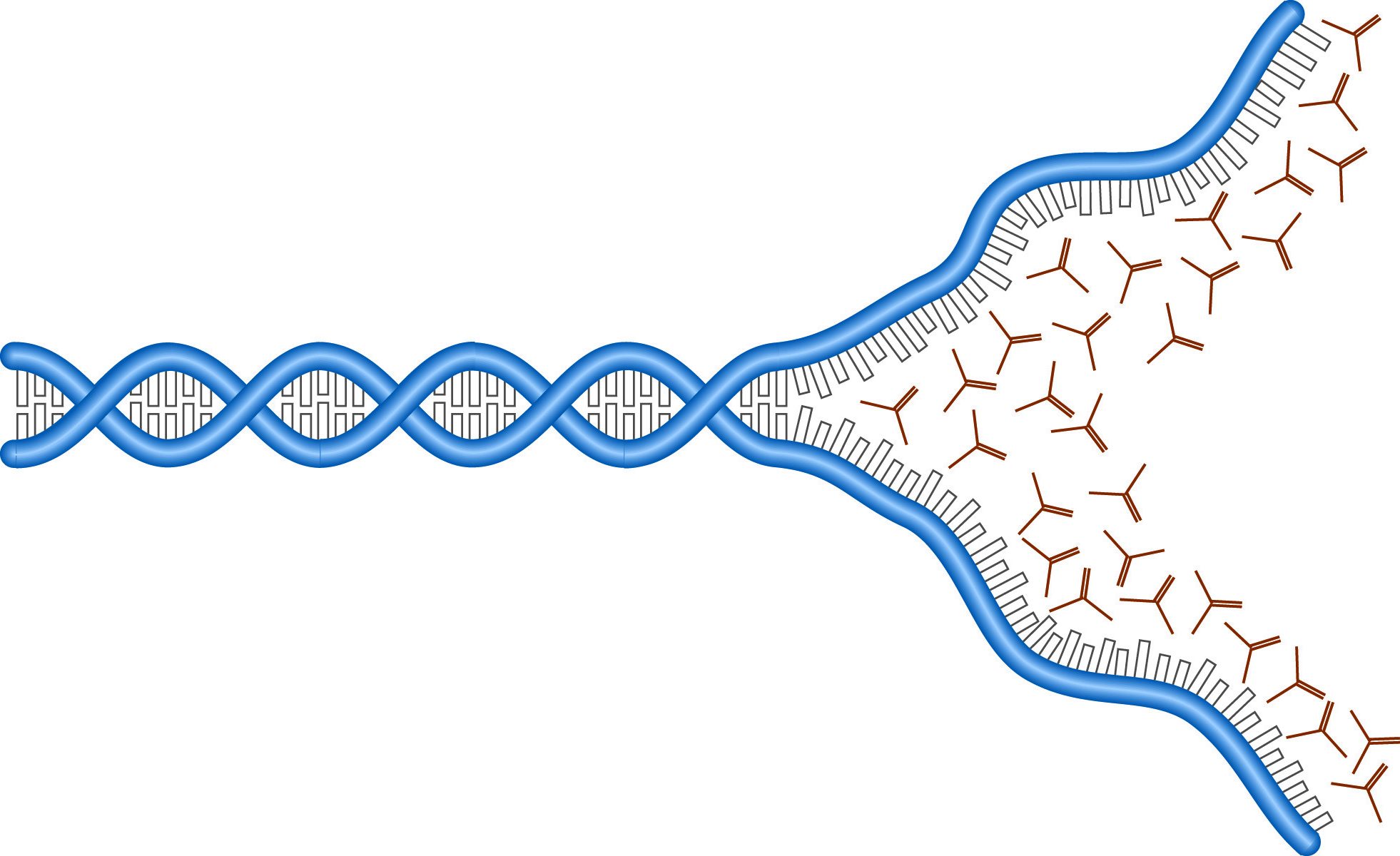
The denaturation of DNA by urea.
The electrophoretic analysis of single-stranded nucleic acids is complicated by the secondary structures assumed by these molecules. Separation on the basis of molecular weight requires the inclusion of denaturing agents which unfold the DNA or RNA strands and remove the influence of shape on their mobility. Nucleic acids form structures stabilized by hydrogen bonds between bases. Denaturing requires disrupting these hydrogen bonds. The most commonly used DNA denaturants are urea and formamide. Each of these forms hydrogen bonds with the DNA bases, "saturating" H-bond sites and preventing the formation of inter-base bonds.
Both formamide and urea effectively lower the melting point of the DNA molecules, allowing the structures to fall apart at lower temperatures. Generally, concentrations of urea or formamide are chosen to give melting temperatures around 50° C, and gels are run at that temperature. RNA is often denatured with harsher agents because RNA tends to form stronger structures. RNA denaturation is discussed in RNA electrophoresis.
NEXT TOPIC: Sample Preparation for Denaturing PAGE of DNA
- Using PAGE to Determine Nucleic Acid Molecular Weight
- SSCP Analysis
- Sanger Sequencing
- Sample Preparation for Native PAGE of DNA
- Sample Prep for Denaturing PAGE of DNA
- S1 Mapping
- Run Conditions in Denaturing PAGE
- RNA Mapping
- RNA Electrophoresis
- Ribonuclease Protection
- Restriction Digest Mapping
- Primer Extension
- Preparing Denaturing DNA & RNA Gels
- Preparation of Denaturing Agarose Gels
- Preparation of Agarose Gels
- Pouring Sequencing Gels
- PFGE and FIGE
- PCR Analysis: Yield and Kinetics
- PCR Analysis: An Examination
- Native PAGE of DNA
- Mobility Shift Assay
- Methylation & Uracil Interference Assays
- Maxam & Gilbert Sequencing
- Manual Sequencing
- In Gel Enzyme Reactions
- Heteroduplex Analysis
- Gel Preparation for Native PAGE of DNA
- Gel Electrophoresis of PCR Products
- DNase I Footprinting
- DNA/RNA Purification from PAGE Gels
- DNA/RNA Purification from Agarose Gels – Electroelution
- Differential Display
- Denaturing Polyacrylamide Gel Electrophoresis of DNA & RNA
- Conformational Analysis
- Automated Sequencers
- Analysis of DNA/Protein Interactions
- Agarose Gel Electrophoresis of DNA and RNA – Uses and Variations
- Agarose Gel Electrophoresis of DNA and RNA – An Introduction
