Post Electrophoretic Analysis Articles
Biological Macromolecules: Nucleic Acids
Nucleic Acids
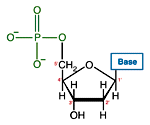
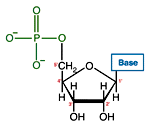
Like many biological molecules nucleic acids are polymers, long molecules formed of repeating units. With nucleic acids, the repeating unit is the nucleotide. A nucleotide consists of a five carbon sugar, a nitrogen containing base and a phosphate group. The two primary kinds of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), possess slightly different sugars in their respective nucleotides and a different set of four bases which may be contained by their nucleotides.
 |
 |
| DNA Nucleotide | |
 |
|
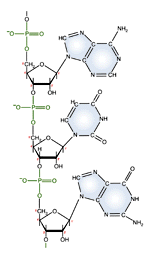
| RNA nucleotide | The structure of a section of an RNA molecule. |
Note the presence of a hydroxyl group on the 2' carbon of the sugar moety.
 |
 |
 |
|
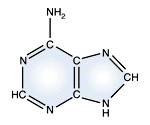
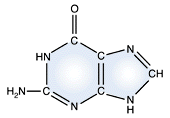
Adenine - DNA and RNA |
Guanine - DNA and RNA |
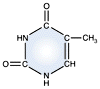
Thymine -
DNA and RNA |
 |
 |
|
|
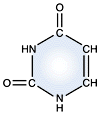
Cytosine - DNA and RNA |
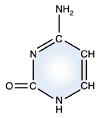
Uracil - RNA only |
Of great importance to electrophoresis is the ionization of the phosphate groups, giving nucleic acids a large net negative charge. Because each nucleotide is ionized, the charge to mass ratio of two different nucleic acid molecules will very closely agree.
 >
>
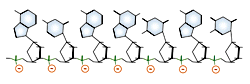
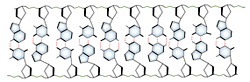
DNA and RNA each contain four possible nucleotides corresponding to the set of four possible bases (adenine, guanine, thymine and cytosine for DNA; adenine, guanine, uracil, and cytosine for RNA). Each base exhibits a particular affinity for one of the other three bases, based on hydrogen bonding symmetries. The nitrogen base adenine "base pairs" with thymine (or uracil in RNA). Guanine "base pairs" with cytosine. Because of base pairing, DNA or RNA can exist as single stranded or double stranded variants. The double stranded form consists of two complementary strands joined by base pairing.
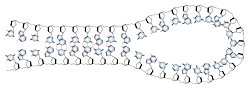
Base pairing can also occur in single stranded DNA or RNA. A section containing one sequence of nucleotides will often loop back and base pair with a complementary section on the same chain. This will affect the 3 dimensional structure of the molecule, with implications for electrophoretic separations. In general, long strands of DNA or RNA will be found in a base-paired conformation, either double stranded or single stranded with internal pairing. Unpaired, or "denatured" nucleic acids are only found in solution under special conditions which destabilize the base pairs.
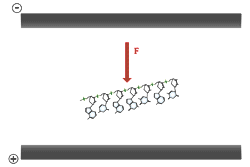
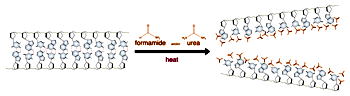
Electrophoresis of double stranded DNA or RNA is referred to as native gel electrophoresis. Electrophoresis of single stranded DNA or RNA occurs under denaturing conditions. Formamide and urea are the two most common agents which accomplish chemical denaturation. These substances act to disrupt the hydrogen bonding between the nitrogen bases, thereby removing the effects of base pairing. Usually some combination of formamide, urea, and heat is employed over the process of denaturing electrophoresis from sample preparation to running the gel. The purposes of denaturing conditions are to ensure single stranded molecules and to prevent conformational changes due to base pairing between different sections of the same DNA or RNA molecule. Denaturing electrophoresis conditions allow for a consistent relationship between molecular size and mobility through the gel.
NEXT TOPIC: Biological Macromolecules-Nucleic Acids
