Post Electrophoretic Analysis Articles
Biological Macromolecules – Proteins
Proteins
Like nucleic acids, proteins are polymers. While with nucleic acids the repeating unit is the nucleotide, with proteins, the analogous repeating unit is the amino acid. Amino acids consist of a central carbon that carries an amino group, a carboxyl group, hydrogen, and a side-chain group. Amino acids are distinguished by the properties of their side chains.
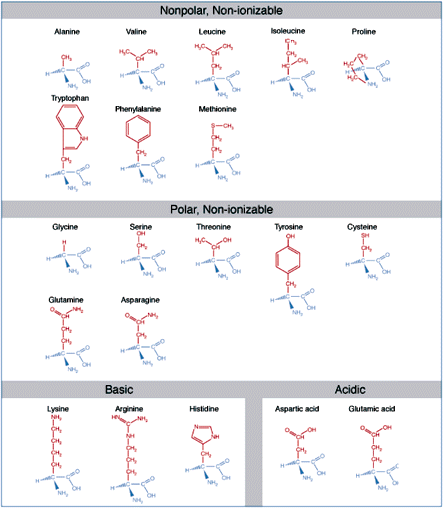
Amino acids are the basic structural units of proteins. An amino acid consists of an amine group, carboxyl group, hydrogen atom, and a side-chain group, all bonded to a central carbon atom. Amino acids are classified according to the solubility properties and ionizability which they derive from their side-chains.
Single chain proteins generally range from 50 to 1000 amino acids in length. When describing protein structure, biologists distinguish primary, secondary, tertiary, and quaternary levels of structure. A protein's primary structure is the actual sequence of amino acids. The secondary structure refers to local bends, kinks, and spirals along the chain. Tertiary structure refers to the shape of the entire polypeptide chain, and quaternary structure is used to describe proteins that consist of more than one polypeptide chain.
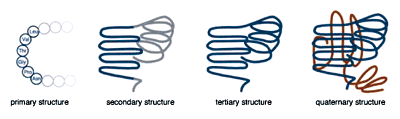
The levels of protein structure.
A protein's state of ionization depends on the nature of its amino acids and the chemical environment. In a neutral, aqueous solution (pH = 7), a protein with a preponderance of basic amino acids, lysine, arginine, or histidine, will have an overall positive charge.
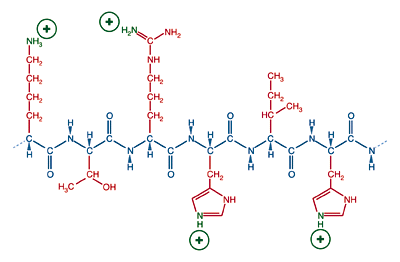
A protein with many basic side chains will have a positive charge and a physiological pH.
Conversely, a protein with many acidic amino acids, glutamic acid or aspartic acid, will have an overall negative charge in neutral solution.
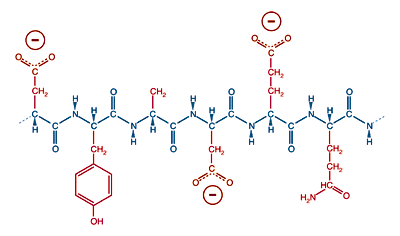
A protein with many acidic side chains will have a negative charge a physiological pH.
The state of ionization depends on the pH of the environment, so almost all proteins placed in a basic environment will accrue a negative charge, losing hydrogen ions as a function of acid/base equilibrium. A protein placed in an acidic environment will tend to become positively charged. Nondenaturing protein electrophoresis is generally carried out in a weakly basic environment. In this environment, most proteins will become negatively charged and migrate towards the positive plate. Denaturing protein electrophoresis, in the presence of sodium dodecyl sulfate (SDS), also causes proteins to obtain a negative charge through emulsification by negatively charged dodecyl sulfate ions (see Buffer Additives).
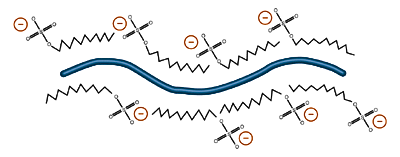
Emulsification by sodium dodecyl sulfate gives proteins a net negative charge. Different proteins in the same SDS solution are imparted with approximately the same charge to mass ratio, an advantage of SDS-PAGE Electrophoresis
The pH where a protein is electrically neutral overall is a function of the type and number of the protein's ionizable groups. At this pH, called the isoelectric point (pI) of the protein, it will not migrate in an electric field. Because the distribution of ionizable groups is different among proteins, they differ in their isoelectric points. This difference is a powerful tool for electrophoretic separation, used in isoelectric focusing.
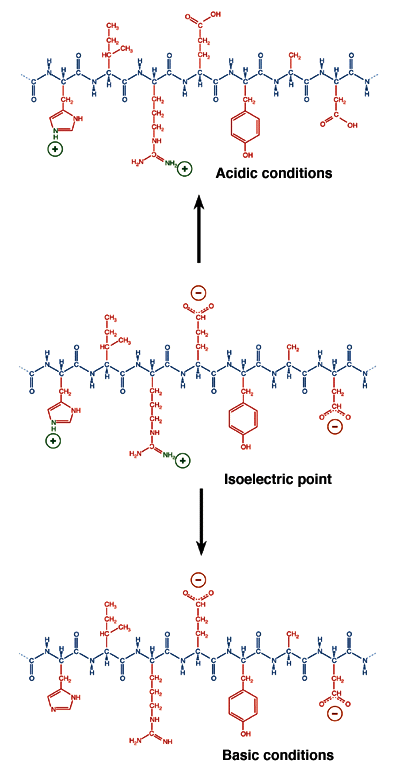
A protein's state of ionization is determined not only by the nature of its side chains but also by the pH of the solution environment. In acidic conditions, proteins tend to acquire a net positive charge. In basic conditions, proteins tend to have a net negative charge. Between these extremes, at a precise value of the pH called the isoelectric point, the value of which is unique for each species of protein, the most thermodynamically stable form of the protein has equal numbers of positive and negative charges and does not migrate in an electric field
NEXT TOPIC: The Mechanical and Electrical Dynamics of Gel Electrophoresis - Intro and Sample Mobility
- The Polyacrylamide Matrix-Buffer Strength
- The Polyacrylamide Matrix
- The Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Ohm’s Law
- The Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
- The Electrophoresis Matrix
- The Agarose Matrix
- Radioactive Emissions and the Use of Isotopes in Research
- Multiphasic Buffer Systems
- Horizontal and Vertical Gel Systems – Vertical Tube Gels
- Horizontal and Vertical Gel Systems – The Vertical Slab Gel System
- Horizontal and Vertical Gel Systems – The Horizontal Gel System
- Homogeneous Buffer Systems
- Faint bands, low background
- Faint Bands, High Background
- Ethidium Bromide Staining
- Electrophoresis Buffers-Choosing the Right Buffer
- Electrophoresis Buffers–The Henderson-Hasselbalch Equation
- Coomassie Blue Stain- Troubleshooting
- Buffer Additives-Surfactants
- Buffer Additives-Reducing Agents
- Buffer Additives-Hydrogen Bonding Agents
- Biological Macromolecules: Nucleic Acids
- Biological Macromolecules – Proteins
