Electrophoresis Buffers-Choosing the Right Buffer
Several factors to consider when choosing a buffer include:
1) pKa value - A buffer should be chosen with a pKa that is very close to the desired pH, preferably within a half point. The buffer will have the greatest capacity both to absorb or release protons with the acid and the base form well represented in solution. It should be noted that pKa is not constant for all conditions but is a function of the total ionic strength and the temperature, so the stoichiometry should be modeled after actual running conditions. Amines are particularly susceptible to changes in pKa with temperature, because, with amines, there is no net increase in ions in solution with the dissociation of ammonium species. Therefore there is little inherent entropy change due to changes in the ordering of water molecules in the solution. This leads to an increase in the significance of the entropy change due to heat flow (the ratio of enthalpy change to temperature) in determining the position of equilibrium.
Usually, in native protein electrophoresis, basic proteins are best separated at acid pH. However, the vast majority of proteins have isoelectric points below 7.5 and are best separated in slightly alkaline conditions, pH 8-9. This pH range has also proven efficacious for most forms of DNA electrophoresis. Thus, buffers with a pKa in the range of 7-9 are best suited for most electrophoretic applications.
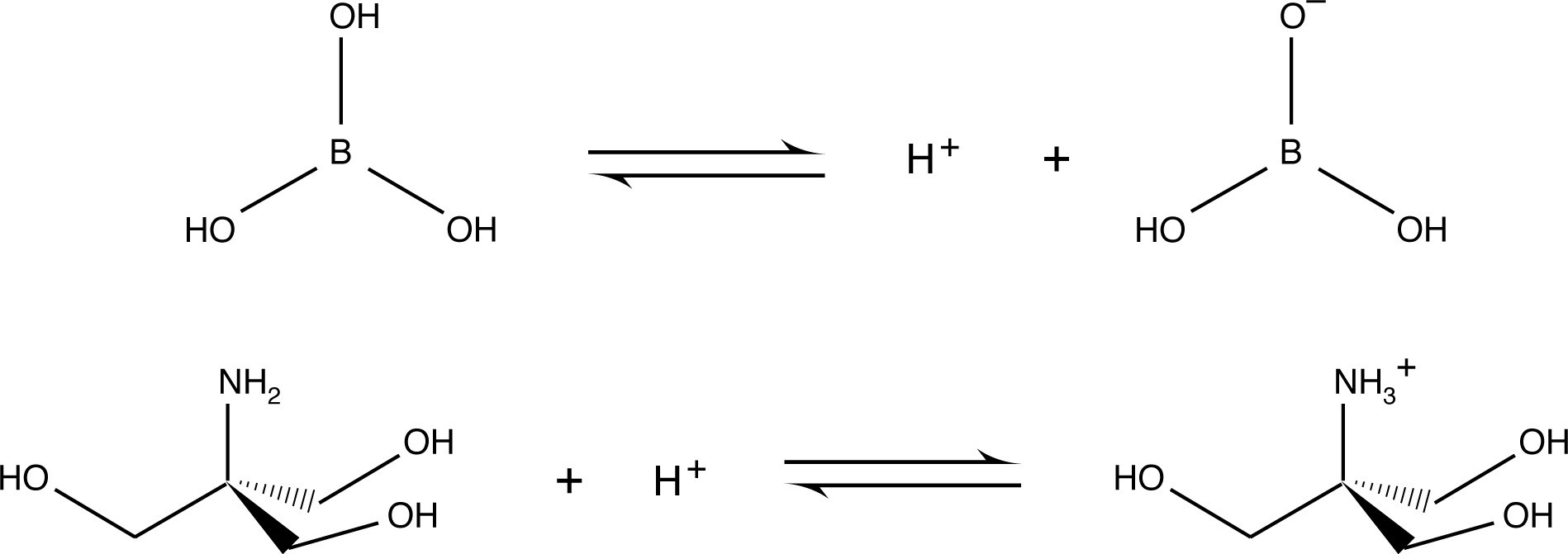
One of the reasons for the relatively low electrophoretic mobility of borate and Tris base is that they are represented by uncharged species in their acid-base equilibrium. It should be further mentioned that the relative electrophoretic mobility of buffer components is a major concern in the design of multiphasic systems, which are discussed in detail in the following section.
2) Formal charges of buffer species - Generally, buffers which form ions of high charge magnitude (+2, +3, -3, etc.) are more difficult candidates with which to work. This type of buffer yields high ionic strength without providing high buffering capacity. At relatively low concentrations, the gel conducts too much current. Furthermore, with ions moving quickly through the gel, the buffer may become depleted. One of the reasons Tris-borate is a popular buffer for electrophoresis is that both Tris base and borate are uncharged part of the time at the desired pH, which reduces their electrophoretic mobility. Reduced mobility of the buffer ions allows for high concentrations of the buffer solution to be employed with the consequent benefits to buffering capacity and sample stability without producing unacceptably high conductivity.
3) Molecular size - In addition to its low charge, Tris base moves slowly in electrophoresis because of its relatively large molecular size. Having a low charge to mass ratio, Tris moves much more slowly than small ions such as chloride or phosphate. Buffer ions are not sieved by the matrix, so their migration rates are determined solely by their charge to mass ratio.
These three factors is by no means exhaustive. Other factors to consider when choosing a buffer would include toxicity, solubility, UV absorption and the possibility of interaction with other species present in the solution.
Among the most commonly used electrophoresis buffers are those shown in the table below:
| Buffer | Molecular Weight | pKa | ΔpKa/oC |
|---|---|---|---|
| Acetic Acid | 60.05 | 4.8 | -0.0002 |
| Boric Acid | 61.83 | 9.23 | -0.002 |
| Citric Acid | 102.1 | 6.4 (pK3) | 0 |
| Glycine | 75.07 | 9.8 | -0.03 |
| MOPS | 209.26 | 7.2 | -0.006 |
| Phosphoric Acid | 98 | 7.2 (pK2) | -0.003 |
| Taurine | 125.1 | 9.1 | -- |
| Tricine | 179.18 | 8.15 | -0.021 |
| Tris | 121.1 | 8.06 | -0.028 |
| Characteristics of buffer salts used in electrophoresis. | |||
NEXT TOPIC: Homogeneous Buffer Systems
