The Buffer
Buffer Additives-Hydrogen Bonding Agents
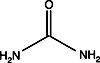
In most forms of electrophoresis, the solution perfusing the gel matrix typically contains one or more substances in addition to the buffer salts. Serving the purpose of modifying the properties of sample molecules, these additives can be categorized as hydrogen bonding agents, surfactants, or reducing agents. Hydrogen bonding agents Urea or formamide can be introduced…
Read MoreElectrophoresis Buffers-Choosing the Right Buffer
Several factors to consider when choosing a buffer include: 1) pKa value – A buffer should be chosen with a pKa that is very close to the desired pH, preferably within a half point. The buffer will have the greatest capacity both to absorb or release protons with the acid and the base form well…
Read MoreElectrophoresis Buffers–The Henderson-Hasselbalch Equation
In its simplest form, a buffered solution contains a mixture of a weak acid and its conjugate base. The position of acid/base equilibrium is represented by the acid dissociation constant, Ka. This number is large if the acid is stronger and equilibrium tends toward dissociation. It is small for an equilibrium that tends toward proton…
Read More