Matrices
Faint Bands, High Background
A high background which obscures the bands, or in combination with fainter than usual bands, indicates dye binding to the gel matrix, or contamination of the matrix with a dye-binding material (most often a protein). Coomassie Blue R-250: Destaining time too short: It takes hours for the dye to completely diffuse out of the gel…
Read MoreFaint bands, low background
Faint bands over a low background with standard Coomassie Blue R-250 staining can be caused by a number of factors: (scroll down for Colloidal Coomassie troubleshooting) SDS in the staining solution: Most users save and re-use their staining solutions. This is fine for a couple of cycles, but over time enough SDS elutes from the…
Read MoreCoomassie Blue Stain- Troubleshooting
If your gel doesn’t look like this one, click on the problem below to find the solution: Faint bands on a low background Learn More Faint bands on a high background Learn More Uneven Staining Learn More Dark Blotches on Gel Learn More Smeared or blurred bands Learn More The ProtoGel Sample Prep Kit gives…
Read MoreEthidium Bromide Staining
Bands in gels stained with ethidium bromide fluoresce under ultraviolet light. The most commonly used stain for detecting DNA/RNA is ethidium bromide. Ethidium bromide is a DNA interchelator, inserting itself into the spaces between the base pairs of the double helix. Ethidium bromide possesses UV absorbance maxima at 300 and 360 nm. Additionally, it can…
Read MoreThe Polyacrylamide Matrix-Buffer Strength
The buffer system in electrophoresis controls the pH of the gel, preventing damage to sample molecules and, in certain cases, controlling the ionization state of the molecules. A second, though no less significant function, derives from the fact that the vast majority of current flowing through the electrophoresis gel is carried by the buffer ions.…
Read MoreThe Agarose Matrix
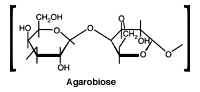
Agarobiose is the basic unit of the agarose. A natural colloid extracted from seaweed, agarose is a linear polysaccharide made up of the repeating unit agarobiose, which consists of alternating units of 1,3-linked b-D-galactopyranose and 1,4-linked 3,6-anhydro-a-L-galactopyranose. Gels prepared from agarose have a substantially larger pore size than polyacrylamide gels. Agarose gels can be prepared…
Read MoreThe Polyacrylamide Matrix
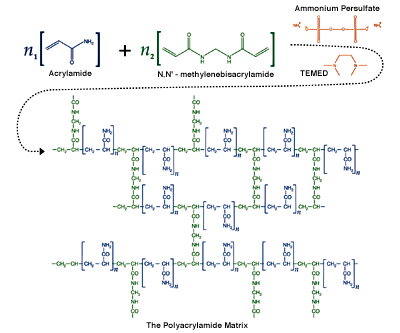
Polyacrylamide gels are formed by the polymerization of acrylamide in an aqueous solution in the presence of small amounts of a bifunctional crosslinker. The crosslinker is usually methylenebisacrylamide (bis, or MBA). The polymerization of a polyacrylamide matrix with methylenebisacrylamide cross-linking. Polyacrylamide gels are formed by the polymerization of acrylamide in aqueous solution in the presence…
Read MoreThe Electrophoresis Matrix
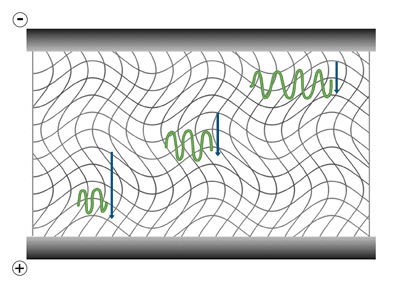
In-gel electrophoresis the matrix forces sample components to separate by size, as they move through its porous structure. The matrix provides greater resistance to the movement of larger molecules. It also performs additional functions including the reduction of convection currents and the inhibition of sample diffusion, encouraging the separated components to remain as sharp, distinct…
Read MoreThe Mechanical and Electrical Dynamics of Gel Electrophoresis — Electrophoresis System Dynamics
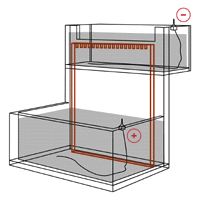
Electrophoresis System Dynamics The apparatus in gel electrophoresis constitutes an electrical/thermodynamic system. The apparatus receives energy from the power source and releases energy as heat. The figure below shows a stylized representation of a typical vertical slab gel apparatus. The gel, perfused with buffer solution and held between two glass plates, has been clamped in…
Read MoreThe Mechanical and Electrical Dynamics of Gel Electrophoresis – Intro and Sample Mobility
The term “electrophoresis” refers simply to the movement of particles by an electric force. The first electrophoresis experiments were carried out on molecules in a conductive buffer solution, where the only force acting on the sample was the electric field. The vast majority of current laboratory techniques are performed in a matrix perfused by an…
Read More