Oxygen Assays Articles
Reactive Oxygen Species
Oxygen is used by a great variety of organisms as a means for producing energy. The redox potential of the oxygen-water couple is 1.229 Volts, meaning that a relatively large amount of energy is released during the 4 electron reduction which converts O2 to H2O. The incorporation of this reaction into cellular metabolism was an enabling step on the evolutionary path to higher organisms. However, harnessing this new resource came at a cost—the intermediates produced during the reduction of molecular oxygen are all highly reactive and pose risks to the cells that use this pathway. The intermediates of Oxygen are shown below:
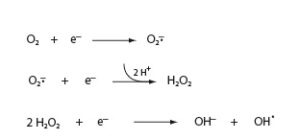
The first reduction product of oxygen is the superoxide radical (O2-). Carrying an unpaired electron, superoxide is a potent oxidizing agent. It has been found to react with numerous cellular structures, such as iron-sulfur clusters, unsaturated lipids, and nucleic acids. Superoxide can also cause biological damage by acting as a reductant, as in the Fenton reaction described below.
Reduction of superoxide yields hydrogen peroxide (H2O2) . Hydrogen peroxide is also a powerful oxidant and is commonly used in first aid as a biocide to cleanse wounds, or in high concentrations as a bleaching agent. While superoxide carries a charge—and is thus unable to freely cross biological membranes—H2O2 is uncharged, and can diffuse across membranes as easily as water. Hydrogen peroxide is also more stable than superoxide, and can diffuse through a cell or tissue, causing damage at a distance from its point of origin.
In the presence of catalytic amounts of iron, superoxide and hydrogen peroxide can react to produce hydroxyl radical (OH.).
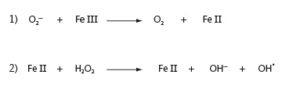
Hydroxyl radical is one of the most potent oxidizing agents known. It is too reactive to diffuse far in a cellular environment rich in targets for oxidation. It is thought to cause damage in the vicinity of iron centers or other sites containing bound iron. Oxidation by hydroxyl radical results in the reduction of the radical to water:
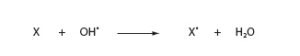
where X is the molecule oxidized by hydroxyl radical.
NEXT TOPIC: Superoxide Detection
