Advanced Histological Techniques
Electron Microscopy
The resolution of a microscope is limited by the wavelength of light passing through the sample. For visible microscopes using 400 nm light (blue light), the limit of resolution is one half the wavelength, or 200nm. This is some two to three orders of magnitude larger than many cellular structures. Electrons, like photons, have wavelike properties, but, unlike light, electrons can be accelerated to wavelengths well below 1nm. This has allowed the development of the electron microscope (EM), with resolutions down to below 1nm. Although electron wavelengths would theoretically allow resolution down to below 0.01nm, in practice, mechanical limitations on the construction of the apparatus have prevented this limit from being approached.
Two types of electron microscopes are in wide use: the transmission electron microscope (TEM) and the scanning electron microscope (SEM). The TEM operates on the same principles as a light microscope. A beam of electrons, accelerated from a tungsten filament, is focused on a sample, and the transmitted electrons are focused into an image. "Electron dense" areas of the sample (often made dense by staining techniques) scatter electrons, leading to dark areas in the image. The image itself, being made up of electrons, is invisible to the eye, so it is visualized by projection onto a fluorescent screen, which emits light when it is struck by electrons. For permanent records, photographic film, which is exposed by electrons, is used in place of the screen.
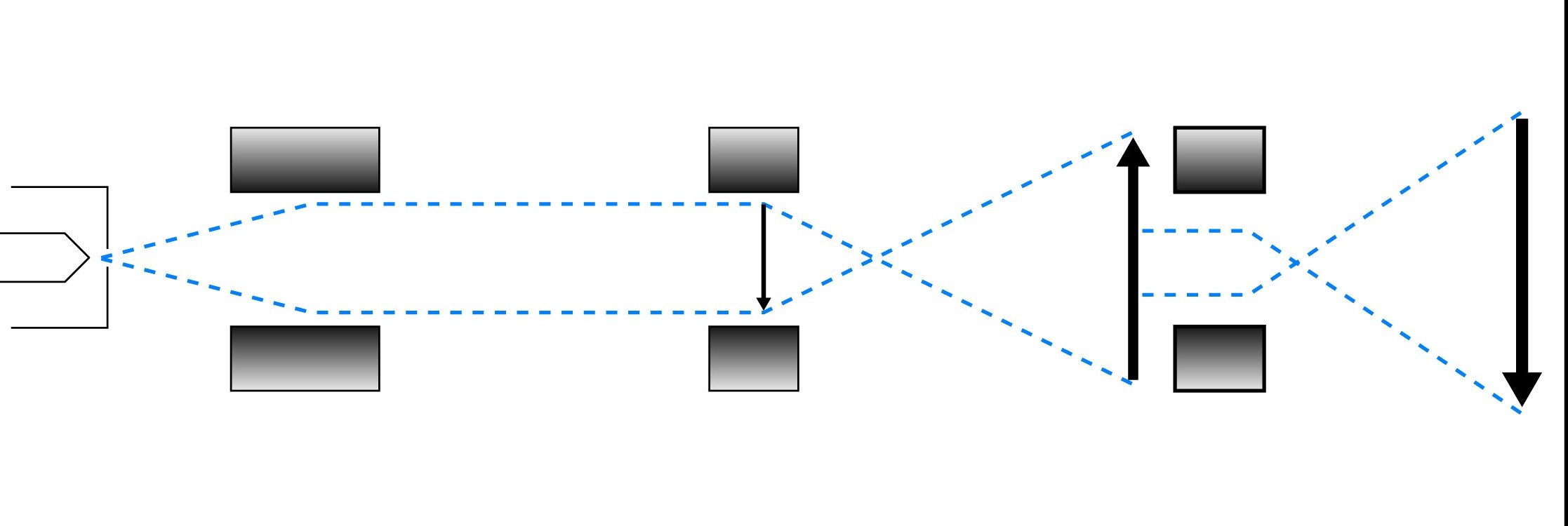
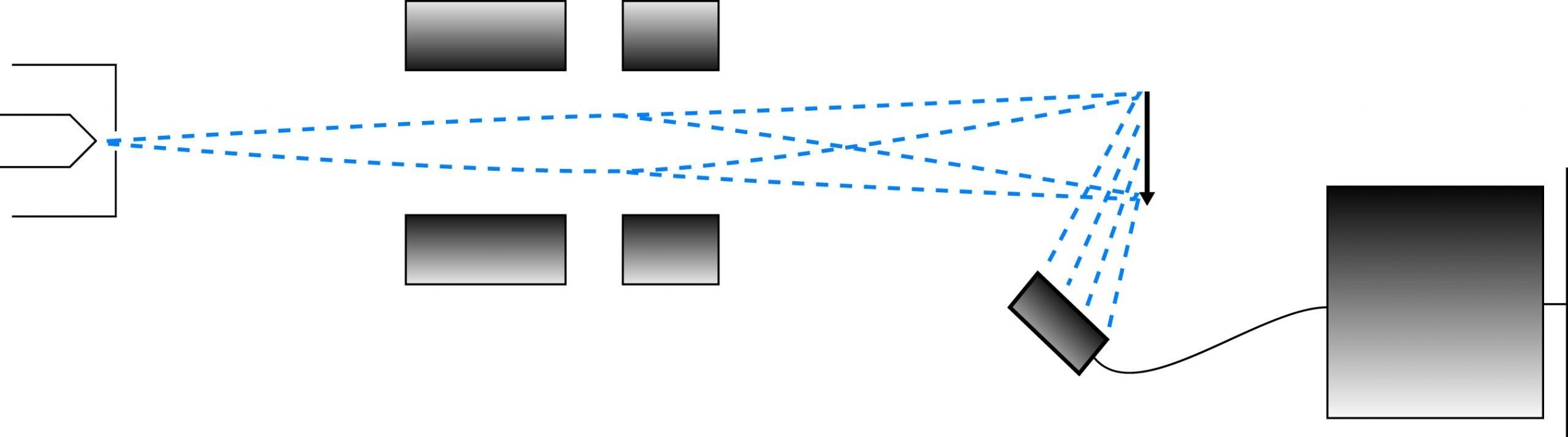
Comparison of the imaging paths of the transmission and scanning electron microscopes.
Of the organelles made clearly visible by the transmission electron micrograph of the hepatic cell above (rat), only the nucleus could have been resolved with a light microscope. Organelles: (I) Nucleus, (II) Endoplasmic reticulum, (III) Mitochondria, (IV) Golgi apparatus, (V) Bile canaliculus, (VI) Plasma membrane, (VII) Desmosome, (VIII) Secretory granule.
The SEM is used to visualize surface details of the sample. The image develops by means of the scattering of electrons by the surface of the sample when the beam hits it. A narrowly focused beam (10nm diameter) is "scanned" across the sample surface, and the secondary electrons which are reflected from the surface are amplified and used to determine characteristics of the sample surface at the probe position.
The preparation of samples for transmission electron microscopy parallels tissue preparation for visible microscopy. Tissue samples must be fixed, processed, embedded, sectioned and mounted before viewing. The sections produced must be thinner and stronger than those for light microscopy, and the level of detail observable mandates that the tissue structure be exceptionally well preserved. Specialized fixing and processing techniques have been refined to meet these requirements. Furthermore, staining techniques have been developed to produce electron dense zones instead of colored or fluorescent areas. (Techniques of sample preparation for scanning electron microscopy are not covered here. For a good overview of the process, the reader is referred to the following resource: Robinson, G & Gray T. (1990) Electron Microscopy 3: Specialized Techniques, in Bancroft, J. & Stevens A. (ed.) Theory and Practice of Histological Techniques, 3rd edn. London. Churchill.)
NEXT TOPIC: Fixing Tissue for Electron Microscopy
