Staining Protein Gels with Coomassie Blue
The Coomassie dyes (R-250 and G-250) bind to proteins through ionic interactions between dye sulfonic acid groups and positive protein amine groups as well as through Van der Waals attractions. Coomassie R-250, the more commonly used of the two, can detect as little as 0.1 ug of protein. Though less sensitive, Coomassie G-250 can be used in place of the R-250 form to create a rapid and convenient staining procedure. This capability of G-250 is due to its particular properties. Coomassie G-250 manifests a leuco form below pH 2. Solutions of the dye, dark blue black at pH 7, turn a clear tan upon acidification. The leuco form recovers its blue color upon binding to protein, apparently due to the more neutral pH of the environment around the protein molecule. Under proper conditions, a gel placed in an acidified solution of Coomassie G-250 will manifest blue protein bands on a light amber background. The bands develop rapidly and there is no need to destain, for the background color is so light as to be essentially clear. This stain is less sensitive than Coomassie R-250 protocols, detecting 0.5 µg of most proteins. The loss in sensitivity is offset by the speed and convenience of the protocol, which saves up to 11 hours versus the most sensitive R-250 procedures.
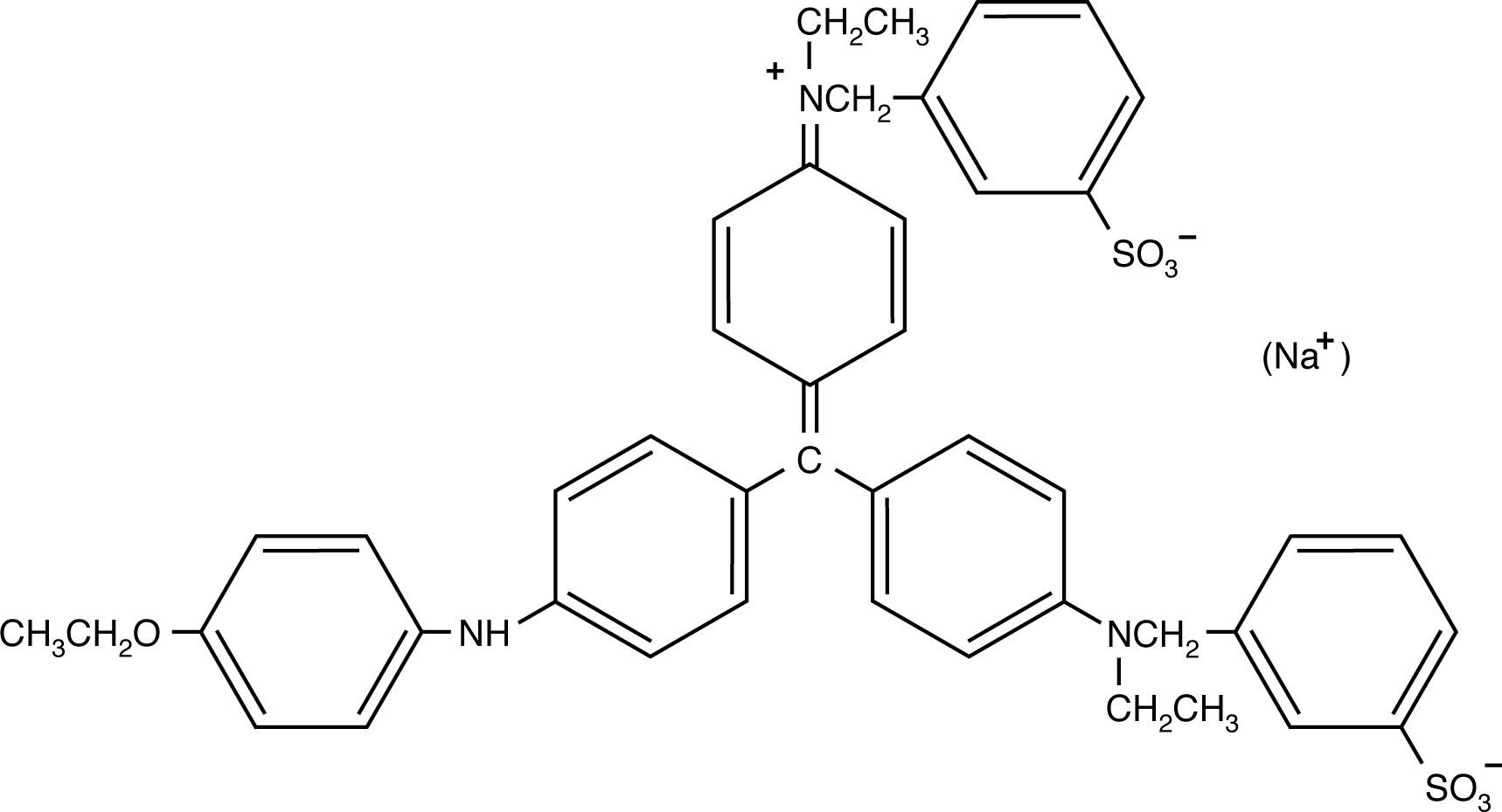
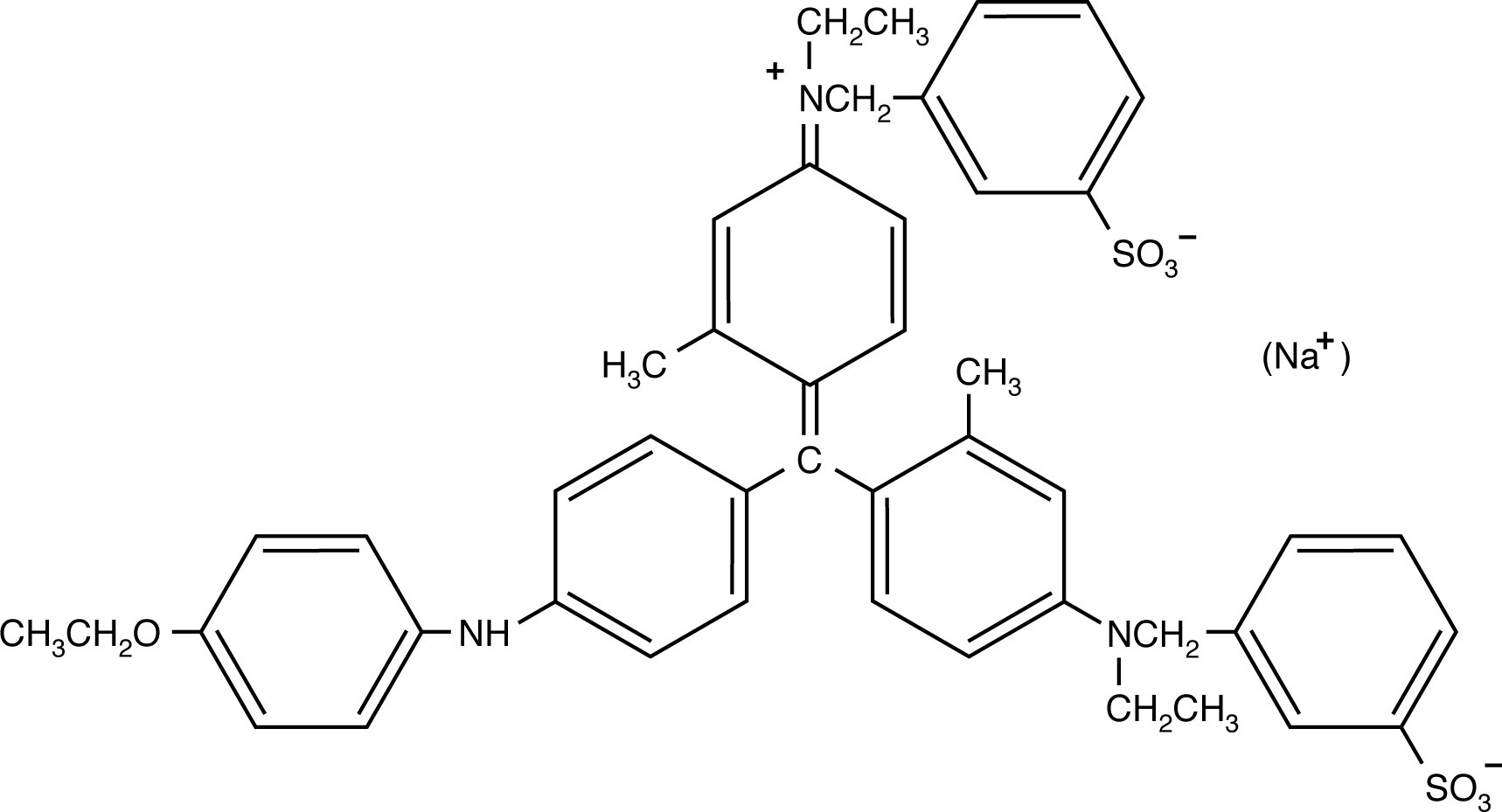 The two Coomassie blue dyes, R-250 and G-250, differ by only two methyl groups. In function, the more popular Coomassie R-250 is more sensitive while G-250 is more convenient.
The two Coomassie blue dyes, R-250 and G-250, differ by only two methyl groups. In function, the more popular Coomassie R-250 is more sensitive while G-250 is more convenient.
Staining Gels with Coomassie Blue R-250 or Coomassie Blue G-250STANDARD PROTOCOL - COOMASSIE BLUE R-250
RAPID PROTOCOL - COOMASSIE BLUE R-250
RAPID PROTOCOL - COOMASSIE BLUE G-250
|
NEXT TOPIC: Silver Staining Protein Gels
