Archive for September 2011
Working Safely with Fixatives
Fixatives are among the most hazardous substances used in life science research. Work with these substances under the hood wearing gloves, lab coat and safety goggles. Formaldehyde is a suspect cancer hazard and a strong sensitizer. It is harmful if inhaled or absorbed through the skin. High exposures may be fatal. Formaldehyde can cause blindness…
Read MoreFactors Affecting Fixation
Fixation protocols are usually straightforward. The tissue is cut to dimensions suited to the rate of penetration of the particular fixative and placed in the fixative solution. The number of factors affecting the fixation process includes buffering, penetration, volume, temperature and concentration. In fixation pH is critical. This is especially the case with formaldehyde, where…
Read MoreNon-Aldehyde Fixatives
Mercury Based Fixatives Mercurials contain mercuric chloride. Their method of tissue fixation is poorly understood. While not penetrating tissue well and causing some tissue hardness, mercurials are fast and provide excellent nuclear detail. They are commonly used to fix hematopoietic and reticuloendothelial tissues. Alcohol Fixatives Alcohols, including methyl alcohol (methanol) and ethyl alcohol (ethanol), are…
Read MoreAssaying Discrete Samples by Liquid Scintillation Counting
Liquid scintillation counting of discrete samples is conceptually straightforward. A sample is mixed with an appropriate volume of scintillation cocktail, and the mixture is placed in an LSC vial and counted. For some samples no additional steps are required, but in many situations samples must be processed to avoid artifacts. The most common causes of…
Read MoreImmunohistochemistry
Immunohistochemistry is the application of antibody/antigen interactions to provide information about biological systems. The body’s response to the introduction of a foreign agent, known as the immune response, results in the production of antibodies which bind the offending material. Antibodies bind tightly and specifically to an “epitope” (one specific structure) on an “antigen” (foreign molecule…
Read MoreAldehyde Fixatives
Formaldehyde and glutaraldehyde are the most commonly used aldehyde fixatives. They work by forming cross-links both within and between proteins, particularly between lysine residues. Damage to the tertiary structure of the proteins occurs on a limited basis. Formalin (37% aqueous formaldehyde) is normally diluted 10 fold and neutrally buffered to make a working fixative solution…
Read MoreOverview of Fixation
To maintain the tissue in as lifelike a state as possible, tissue for analysis is usually placed directly into a fixative solution upon removal from the body. Fixation is normally carried out as soon as possible to prevent autolysis and to reduce possible infectivity. Several factors determine the choice of fixative for a given application.…
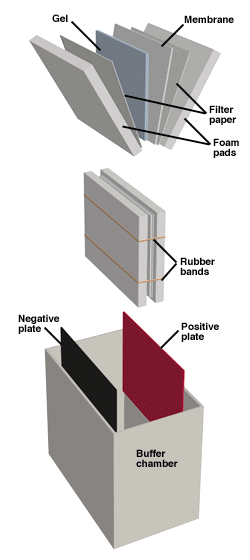
Read MoreOverview of Western Blotting
Proteins can also be detected immunologically following electrophoresis, a technique known as Western blotting. This method relies on the fact that most epitopes (sites recognized by antibodies, generally comprising several amino acids) are still recognizable following denaturing of the protein with SDS and binding to the surface of a membrane. The Western Blotting apparatus. Proteins…
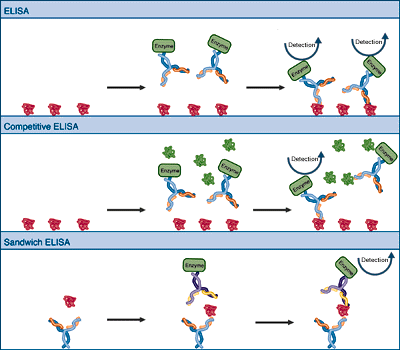
Read MoreEnzyme Linked Immunosorbent Assay (ELISA)
One of the most straightforward applications of immunological detection is the ELISA or enzyme-linked immunosorbent assay. In the simplest system, the bound antigen is probed with antibodies that carry covalently attached enzyme molecules. Antibody binding immobilizes the enzyme in the vicinity of the bound antigen, allowing detection of the antigen. Variations include a competition ELISA…
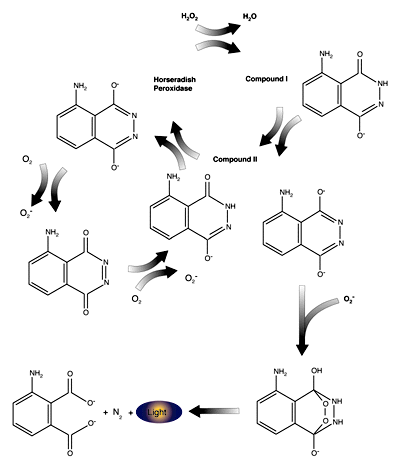
Read MoreImmunostaining with Horseradish Peroxidase
Chromogenic and luminometric substances are also available for horseradish peroxidase (HRP). The “classic” chromogen used with HRP is diaminobenzidine (DAB). In the presence of H2O2, HRP will oxidize DAB, creating a water insoluble brown precipitate. DAB has two primary disadvantages, it is relatively insensitive and it is a potential carcinogen which must be decontaminated before…
Read More